Background and aims
Myeloid malignancies encompass a diverse group of conditions, including myelodysplastic neoplasms (MDS) and chronic myelomonocytic leukemia (CMML). There is evidence suggesting the role of bone marrow (BM) environment and immune cells in the development and progression of MDS and CMML. Azacytidine (AZA) remains the backbone therapy for higher-risk MDS and non-proliferative CMML. To date, molecular predictors of response to AZA are not yet well-defined. Herein, we sought to investigate the immune landscape of BM samples from patients with lower-risk MDS (LR-MDS; i.e., IPSS-R <3.5), CMML, higher-risk MDS (HR-MDS; i.e., IPSS-R ≥3.5), and acute myeloid leukemia (AML).
Methods
Samples were collected before treatment initiation. Mass cytometry (CyTOF) was performed on isolated BM mononuclear cells from 48 patients, using the Maxpar® Direct™ Immune Profiling Assay. Flow cytometry was performed on a secondary cohort of 56 patients. scRNA-sequencing was performed on sorted BM CD8+ T cells from patients with HR-MDS (n=4) and AML (n=5), using a 10x Genomics pipeline. Mann-Whitney U test and the Kruskal-Wallis test were used as appropriate. Kaplan-Meier analysis was utilized for survival analysis, and the log-rank test was employed to assess the significance. The level of significance was established at P < 0.05.
Results
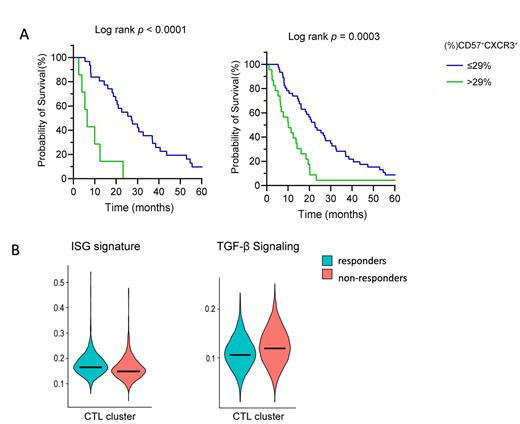
The immunophenotypic analysis using CyTOF demonstrated increased expression of CXCR3 on cytotoxic CD8+ T-cells in CMML and AML samples compared to samples from patients with MDS. Increased frequency of the CD57 +CXCR3 +CD8 + T-cell subset was further observed in patients with HR-MDS and AML that were non-responders to AZA, compared to responders, as observed by both CyTOF and flow cytometry. After determining the optimal cut-off for the frequency of CD8 +CD57 +CXCR3 + T cells at 29%, we observed that HR-MDS and AML patients (Figure 1A) with a frequency of CD57 +CXCR3 +CD8 + of <29% had better overall survival (OS).Multivariate analysisshowed that a frequency of CD57 +CXCR3 +CD8 + of >29% was independently associated with decreased survival in patients with TET2 mutations (HR=2.603; 1.275-5.317). scRNA-seq analysis of isolated BM CD8 + T cells was further performed to characterize the molecular signature that favors response to AZA, which resulted in the identification of several cell clusters based on gene expression. The cluster characterized by the expression of genes associated with cytotoxicity showed significant differences between responders and non-responders. Specifically, within the cytotoxic cluster, genes associated with IFN-related pathways were upregulated in cells from responders to AZA. In contrast, genes upregulated in non-responders displayed an enrichment for the TGF-β signaling pathway, which is known to be associated with the suppression of the killing capacity of T cells (Figure 1B). The aforementioned molecular signatures were further associated with a higher cytotoxic molecular signature in the cytotoxic cell cluster from the group of responders.
Conclusion
In this study, we focused on BM CD8 + T cells in samples from patients with myeloid neoplasias and performed deep phenotypic analysis using CyTOF coupled with scRNA, in order to characterize the BM immune environment. We demonstrate for the first time that BM CD8 + T cells show different phenotypic and transcriptomic characteristics in responders to AZA. Specifically, we show that the frequency of the effector CD57 +CXCR3 +CD8 + subset is decreased in responders before treatment initiation, and this is associated with better survival. Despite the decreased proportion of this cytotoxic cell population in responders, there are specific signatures associated with effector T cell functionality that can explain the improved outcomes in these patients. These findings underscore the significance of the crosstalk between leukemic and immune cells in the progression of the disease and the effect of treatment.
Figure legend
A. Increased (%) CD57 +CXCR3 + correlates significantly with worse survival of HR-MDS and AML patients. Kaplan Meier curves for Overall Survival in HR-MDS and AML patients respectively. B. Gene signature derived from the scRNAseq analysis. Interferon-stimulated gene (ISG) signature is increased in the cytotoxic cell cluster in responders and and TGF-β signature in non-responders.
Disclosures
Kotsianidis:Novartis: Consultancy, Research Funding; Bristol: Consultancy; Genesis: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria.